Helix Chromatography, Inc.’s reliable and fast LC method development and testing services using proprietary mixed-mode HPLC columns can provide you with timely, accurate, and precise results for your analytical needs.
Our mixed-mode HPLC columns have hydrophobic, hydrophilic, and ionic properties, which allow them to retain and separate a wide range of analytes with high selectivity and sensitivity. Our experienced team of chromatography scientists can develop LC methods tailored to your specific needs, from small molecules to large biomolecules.
Our LC method development process involves screening different mixed-mode columns (HILIC mixed-mode and reversed-phase mixed-mode) optimizing chromatography conditions such as buffer nature, buffer concentration, and buffer pH, with various amounts of organic in the mobile phase. This ensures that the method is robust, efficient, and reproducible. When you use multiple mechanisms of interactions, like in multi-mode chromatography you can achieve better and faster separation compared to single-mode columns/methods.
Our company can develop methods within days if not hours and will provide you with the necessary information on the sample preparation, method setup, and analysis, and explain to you why specific columns or conditions were used, this will help you to become an even better expert in method development than you are now.
HELIX Chromatography provides several options for Method Development Services
Experience scientists will be assigned to the project to develop your methods. We will sign a Non-Disclosure Agreement if your company requires this. Once we have details for your separation we can estimate the time required for the method and let you know when we can start on your project. You have the right to reject our recommendation and decide on your own what service to use. Once we develop a method we will ask you to review it and let us know if anything else needs to be done. We will use our columns to develop a method, but there is no obligation to buy any columns.
We try to start method development within two days of receiving samples, but we can also accommodate rush requests. If we have the required compounds in our library we can use them to develop a method for you, otherwise, you’ll need to provide samples and standards. Providing us with samples that are not in solution can help us develop the method much faster. We require 5-10 mg of each standard/marker. We require the submission of standards or markers to identify peaks in your specific mixture.
We provide various tiers of service:
- Basic Free MDS The basic free screening method development includes screening done with 2 columns and 2 different mobile phases. The total time allowance will not exceed 3 hours and will be scheduled as time permits. Chromatograms and raw data may be provided if needed. We reserve the right to terminate the project if the time is exceeded or ask customers to move to a paid tier. The customer is updated when the project is completed.
- Silver MDS Paid Method Development Services. Screening as many as 5 columns with 3 mobile phases. The total time allowance is 8 hours at $225/h. Daily updates on the project upon request. After 8 hours the customer has a choice to terminate the project or move to the next tier option. Providing chromatograms in PowerPoint/PDF format, and raw data per request.
- Gold MDS Paid Extended Method Development Services. Includes finding optimum conditions achieving desired and specified resolution between compounds. Time allowance is up to 40 hours at $275/h. After 40 hours the customer has a choice to terminate the project or move to the next tier option. Daily updates on the project upon request. Providing reports, chromatograms, raw data, and recommendations.
- Diamond MDS Pre-Validation Method Development Services. Includes finding optimum conditions, and achieving desired and specified resolution between compounds. Testing 3 different lots of columns to provide reproducibility data. Conducting quantitation and linearity studies. Time allowance varies on the project. Daily updates on the project upon request. Providing reports, chromatograms, raw data, and recommendations. 30% discount for the first 3 columns upon receiving the payment for method development
Mixed-Mode Method Development Kits
Method development kits below can be used to develop methods for all your compounds. Initial experiments on 50 mm columns can be scaled up on 150 mm columns in Screen and Resolve HILIC and RP Mixed-Mode Kits.
Steps in our method development services:
1) Learn the structure and properties of analytes and identify the detection technique most suitable for the task as well as a set of columns for method development screening.
2) Identify if sample preparation, beyond dissolution and dilution is needed. Proper sample preparation is critical for successful chromatography testing. It is important to use appropriate solvents and buffer systems to ensure the sample is properly dissolved and to minimize any interference with the chromatography column. For mixed-mode chromatography, it is also important to select the appropriate elution buffer that will allow for the separation of the desired compounds.
3) Column selection: Choosing the right mixed-mode chromatography column is crucial for achieving optimal separation. Different mixed-mode columns have different chemistries, and the choice of the column should be based on the specific characteristics of the analyte(s) functional groups and the mechanism of interaction which are present in the column. The broad range of our mixed-mode chemistries offers an unprecedented approach to method development.
4) Optimization of chromatography conditions: The optimization of chromatography conditions is key to obtaining accurate and reliable results. Our approach is to achieve the best resolution possible. This will help to maintain the desired separation even in case of column contamination and lot-to-lot variability of mixed-mode silica gel. Our lot-to-lot variability is tested on 10 different compounds with various mobile phases to ensure that the variability does not exceed 3%. We can also reserve specific lots if customers would like to do that.
5) Perform separation of standards with the optimal conditions.
6) Offer customers either method transfer or analyze customer samples according to the developed method.
7) Communicate results via a short report.
8) Receive feedback and address any possible issues.
Our state-of-the-art analytical laboratory is equipped with the latest chromatography HPLC system equipped with multiple detectors (UV DAD, ELSD, CAD, RI, MS), allowing us to deliver high-quality results in a timely manner. We know mechanisms of interaction and we know how to control them in order to achieve the most efficient separation and resolution. We pride ourselves on our commitment to customer satisfaction and offer flexible and competitive pricing.
If you are interested in our LC method development and testing services, please contact us to discuss your analytical needs and how we can assist you in achieving your goals.
Examples of Method Development Projects
 |
HPLC Method Development and Revalidation for Analysis of Fosfomycin Trometamol Oral Solution
Request more information about analysis of fosfomycin trometamol oral solution. |
 |
HPLC Method Development for Analysis of Arginine-Polyamine Metabolites Cycle
Request more information about analysis of arginine-polyamine metabolites cycle. |
 |
HPLC Method Development for Analysis of Empagliflozin-Metformin Diabetes Drug Combo
Request more information about analysis of empagliflozin-metformin diabetes drug combo. |
 |
HPLC Method Development for Analysis of Trijardy Diabetes Drug Combo Empagliflozin-Linagliptin-Metformin
Request more information about analysis of trijardy diabetes drug combo empagliflozin-linagliptin-metformin. |
 |
HPLC Analysis of Hemp Oil and CBD Isolate for CBD and THC Content
BD Distillate is a distilled extract derived from hemp biomass (flower and plant). CBD distillate usually contains 80%+ of CBD with the rest being other minor cannabinoids, terpenes, and other plants oils and extracts. The federal regulations call for tetrahydrocannabinol to be less than 0.3%. We have developed an HPLC procedure for analysis of CBD and THC in hemp products. This method can be applied to analysis of all cannabinoids found in hemp and marijuana plants. The same methodology can be used for your cannabis analysis.Request more information about analysis of hemp oil and CBD isolate for CBD and THC content. |
 |
HPLC Analysis N-Nitrosodiethylamine in Valsartan Drug Formulation
In recent months several blood pressure medications were recalled for the presence of potentially cancerogenic impurity – N-Nitrosodiethylamine. We have developed a procedure for quantitative analysis of N-nitrosamines in blood pressure formulations. You can use our columns and method for analysis of impurities and API in drugs and drug formulations.Request more information about analysis of n-nitrosodiethylamine in valsartan drug formulation. |
 |
HPLC Analysis of Trace Levels of Paraquat and Glyphosate in Fruits and Vegetables
Paraquat and glyphosate are two of the most widely used herbicides world wide. Both compounds are very polar, ionic, with an opposite charge. Paraquat is a hydrophilic highly basic compound and glyphosate is polar acidic compound. We have developed stationary phase and method for simultaneous analysis of these two herbicides in various matrices, including fruits, vegetables, drinking and waste waters. The approach and column are suitable for analysis of glyphosate, paraquat and other herbicides/pesticides in different samples.Request more information about analysis of trace levels of paraquat and glyphosate in fruits and vegetables. |
 |
HPLC Analysis of Process Waste Water in Biodiesel Production. Part 1. Phosphate Ion and Total Organic Analysis
We have developed a new approach to analysis of total organic matter and phosphate ion in the wastewater in biodiesel production. A short 3-minute method allows to quantify organic components and phosphate ion levels in wastewater streams. The method is robust and LC/MS compatible. Amaze N1 mixed-mode column was used to retain and separate target compounds.Request more information about analysis of process waste water in biodiesel production. |
 |
HPLC Analysis of Filter Deposits from Biodiesel Production
Biodiesels are produced in large quantities using transesterification of animal and vegetable fats/oils. Optimization of the manufacturing cost is directly related to successful and economically viable use of biodiesel. One of the bottlenecks of the production is the issue with filtration and buildup on the filter during the production, which significantly slows down the entire filtration process. Up to now, there has been no study on what causes the drastic slowdown of filtration during biodiesel production. We have conducted such a study on the filter deposits and were able to develop a group analysis for components of biodiesel adsorbed on fiberglass filters. Active measures to minimize these deposits were implemented, increasing the rate of filtration. Same methodology can be used for analysis of your biodiesel production. A selective mixed-mode HPLC method for analysis of glycerides, triglycerides, phospholipids and fatty acids present in raw material was developed.Request more information about analysis of biodiesel, glycerides, triglycerides, phospholipids and fatty acids. |
 |
Development of Stability Indicating HPLC Method for Analysis of Metal ChelatesRequest more information about analysis of biodiesel, glycerides, triglycerides, phospholipids and fatty acids. |
 |
Comprehensive Study of Carryover in HPLC Analysis of Paraquat and DiquatRequest more information about analysis of biodiesel, glycerides, triglycerides, phospholipids and fatty acids. |
Helix Chromatography Testing Services
HPLC testing services with mixed-mode columns can be used to analyze a variety of compounds, including small molecules, peptides, proteins, and nucleic acids. These services are commonly used in the pharmaceutical, biotechnology, and food industries for quality control, purity analysis, and quantification of compounds. We have one of the most comprehensive sets of analytical and preparative columns in the industry.
The benefits of using mixed-mode columns in HPLC testing services include improved selectivity, increased resolution, and greater sensitivity. Mixed-mode columns can also reduce the need for multiple columns, which can save time and resources in method development and testing. The same column can be used in single or multiple modes depending on the nature of the compounds and the composition of the mobile phase.
Examples of Our Testing Services
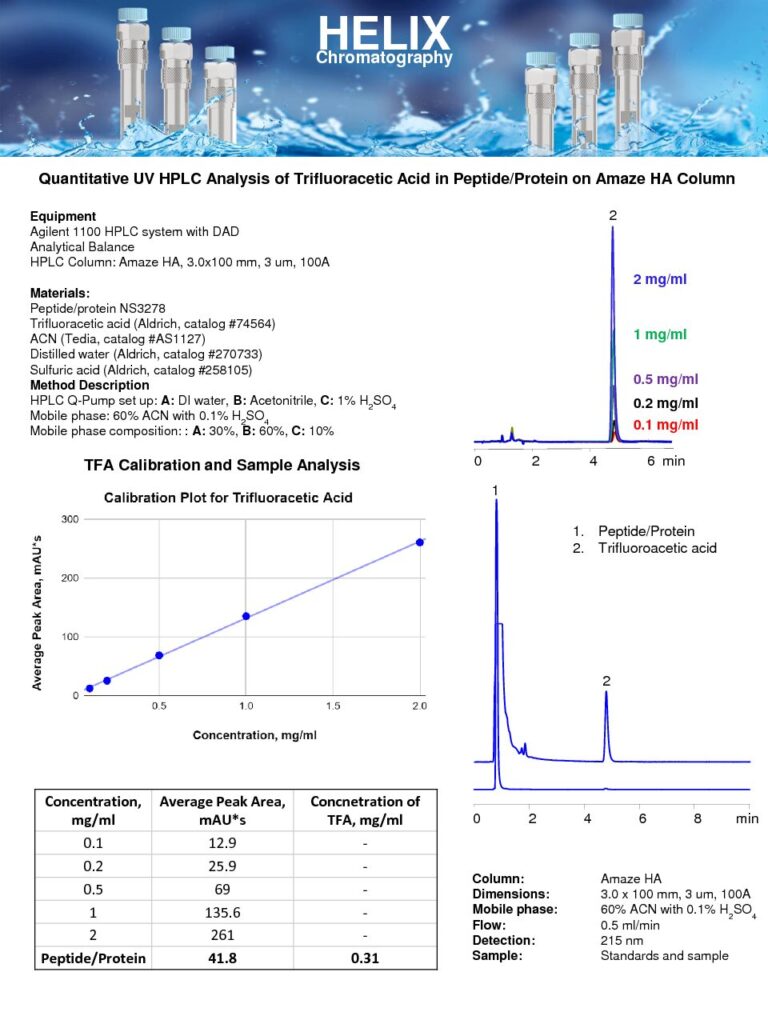 |
Quantitative UV HPLC Analysis of Trifluoroacetic Acid in Peptide-Protein
The Quantitative UV HPLC Analysis of Trifluoroacetic Acid in Peptide-Protein on Amaze HA mixed-mode column was developed, as part of our HPLC Testing Services, to measure the concentration of trifluoroacetic acid (TFA) in a sample containing peptide-protein compounds.The UV detection method is utilized to quantify TFA due to its relatively strong absorbance at lower wavelengths. The Amaze HA mixed-mode column facilitates the retention and resolution of the TFA compound. Protein and peptides are retained by reversed-phase and strong cation-exclusion mechanisms. Trifluoroacetic acid is retained on Amaze HA by a strong ion-exchange mechanism. All compounds retained by reversed-phase and cation-exchange mechanisms can be eluted closer to void, allowing for easy quantitation of TFA.This quantitative analysis is crucial in peptide-protein research, as TFA is commonly used as an acidifying agent during peptide synthesis and purification processes. Accurate determination of TFA levels is essential for ensuring the quality and purity of peptide-protein compounds. The UV HPLC analysis on the Amaze HA mixed-mode column provides a sensitive and robust method for precise TFA quantification, contributing to the overall characterization and understanding of peptide-protein samples. |
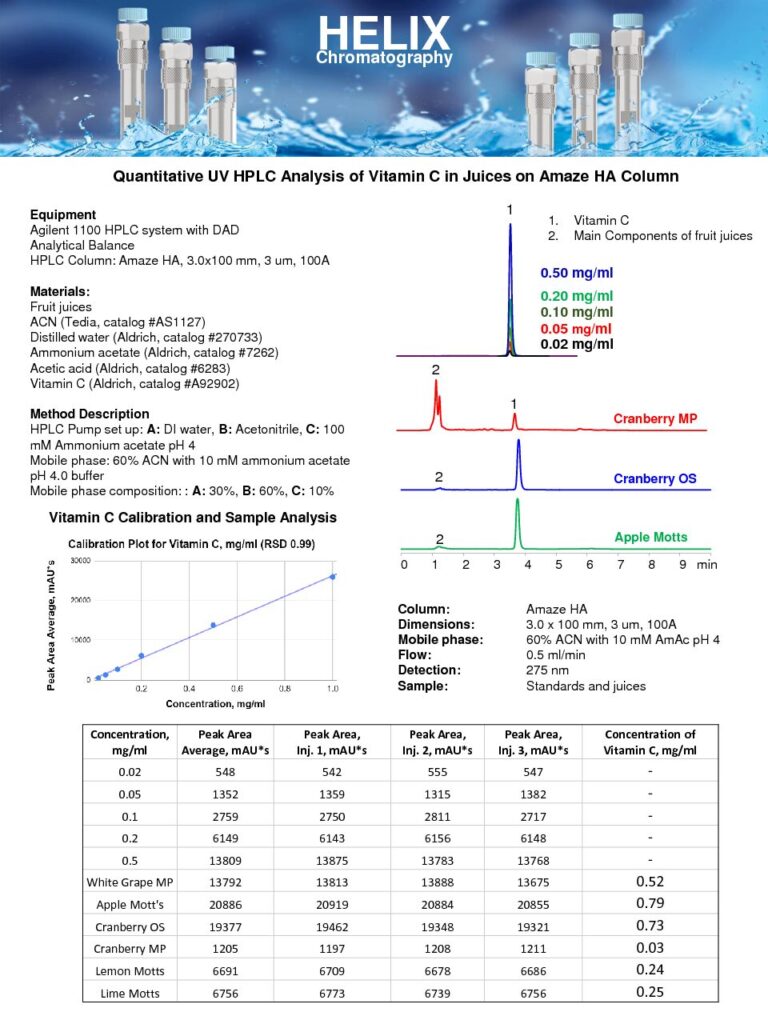 |
Quantitative UV HPLC Analysis of Ascorbic Acids in Juices
The Quantitative UV HPLC Analysis of Ascorbic Acids in Juices on Amaze HA mixed-mode column was developed, as part of our HPLC Testing Services, to measure the concentration of ascorbic acid (vitamin C) in various juice samples.The UV detection method is utilized to quantify ascorbic acid due to its strong absorbance in UV. The Amaze HA mixed-mode column enhances the retention of ascorbic acid by an anion-exchange mechanism, ensuring reliable and reproducible results. Other components of the juices can be eluted earlier to provide accurate quantitation of ascorbic acidThe quantitative analysis of ascorbic acid in juices is essential for determining the nutritional quality and freshness of the beverage. Ascorbic acid is a crucial antioxidant and plays a significant role in the human diet. Measuring its concentration helps assess the vitamin C content in juices, enabling manufacturers to label their products accurately and consumers to make informed choices.
This method provides a sensitive and robust approach for quantifying ascorbic acid in various juice samples. It contributes to the quality control and nutritional evaluation of juices, ensuring they meet the desired standards and contain adequate levels of vitamin C. |
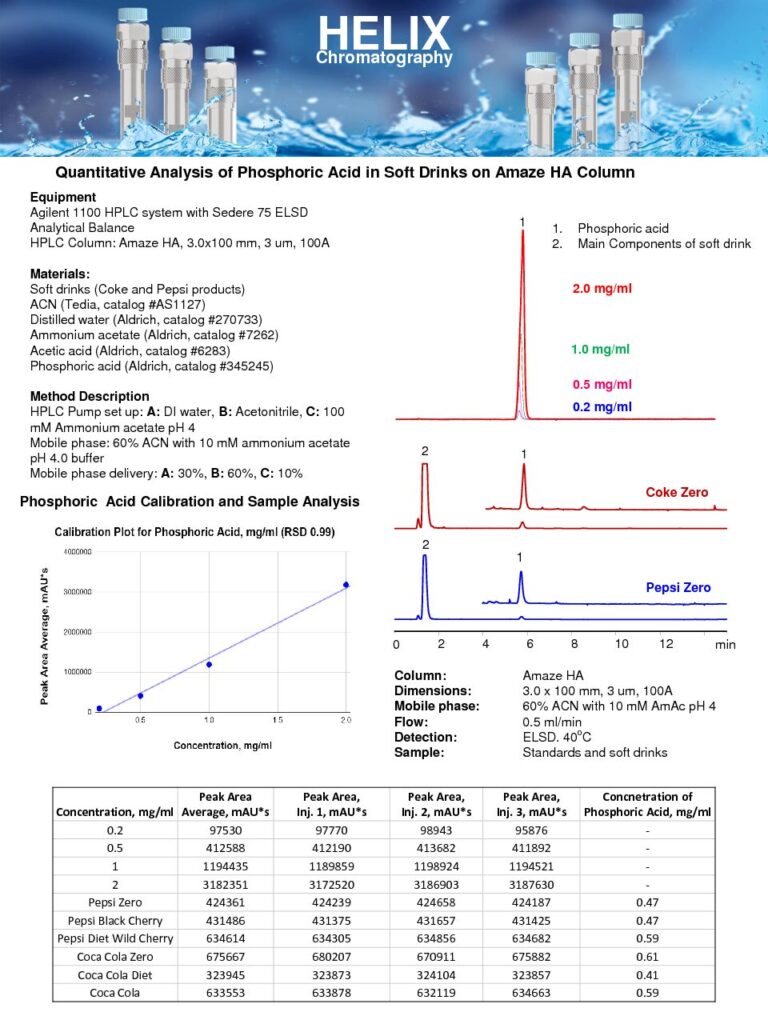 |
Quantitative HPLC Analysis of Phosphoric Acid in Soft Drinks
The Quantitative HPLC Analysis of Phosphoric Acid in Soft Drinks on Amaze HA mixed-mode Retention column was developed, as part of our HPLC Testing Services, using Evaporative Light Scattering Detector (ELSD).Phosphoric acid is commonly used in the production of soft drinks for several reasons. It provides a tart and acidic flavor, contributing to the overall taste profile of many carbonated beverages. Additionally, phosphoric acid acts as a pH regulator, helping to balance the acidity of the drink and provide a desirable level of tanginess.The HPLC analysis method allows for accurate and precise measurement of phosphoric acid levels in soft drinks. The Amaze HA mixed-mode column retains phosphoric by a strong anion-exchange mechanism. time of phosphoric acid can be adjusted by the amount of the buffer and buffer pH. Other components of soft drinks can be moved away from phosphoric acid by changing the amount of acetonitrile in the mobile phase. By utilizing a quantitative HPLC approach, the concentration of phosphoric acid can be determined with high sensitivity and reproducibility.
The measurement of phosphoric acid content in soft drinks is important for various reasons. It allows manufacturers to ensure that the desired flavor profile and acidity of their products are maintained consistently. Furthermore, it provides regulatory authorities with information regarding the composition and labeling of soft drinks, enabling consumers to make informed choices about their beverage consumption.
Through the use of the Amaze HA mixed-mode column in HPLC analysis, this method provides a reliable and accurate means of quantifying phosphoric acid in soft drinks. It contributes to quality control measures and regulatory compliance in the soft drink industry, ultimately ensuring that consumers can enjoy beverages that meet their taste preferences and nutritional needs. |
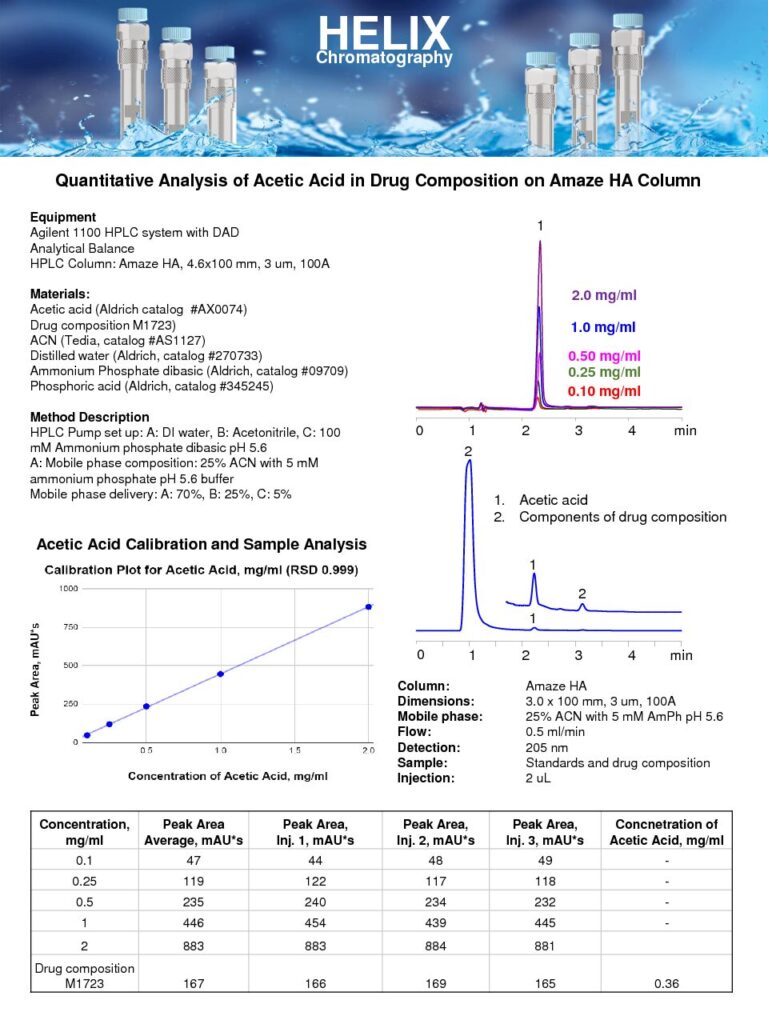 |
Quantitative UV HPLC Analysis of Acetic Acid in Drug Formulation
The Quantitative UV HPLC Analysis of Acetic Acid in Drug Formulation on Amaze HA mixed-mode column was developed, as part of our HPLC Testing Services, to measure the concentration of acetic acid in drug formulation.Acetic acid is a commonly used component in drug formulations, serving various purposes such as pH adjustment, stabilization, and solubility enhancement. However, accurate determination of acetic acid levels is crucial to ensure the safety and efficacy of the drug product.The UV detection method is employed to quantify acetic acid at low UV wavelengths. The mixed-mode approach allows for the efficient separation of acetic acid from the drug formulation components, enabling accurate measurement. The Amaze HA mixed-mode column enhances the retention and resolution of acetic acid by a weak anion-exchange mechanism.
The quantitative analysis of acetic acid in drug formulations provides valuable information for quality control and regulatory compliance. It helps ensure that the drug product contains the desired amount of acetic acid, maintaining its stability and desired properties. Additionally, accurate measurement of acetic acid levels assists in assessing potential impurities or deviations from the specified formulation.
By employing UV HPLC analysis on the Amaze HA mixed-mode column, this method provides a sensitive and robust approach for quantifying acetic acid in drug formulations. It contributes to the overall characterization and quality assessment of drug products, ultimately ensuring their safety and efficacy for patients. |
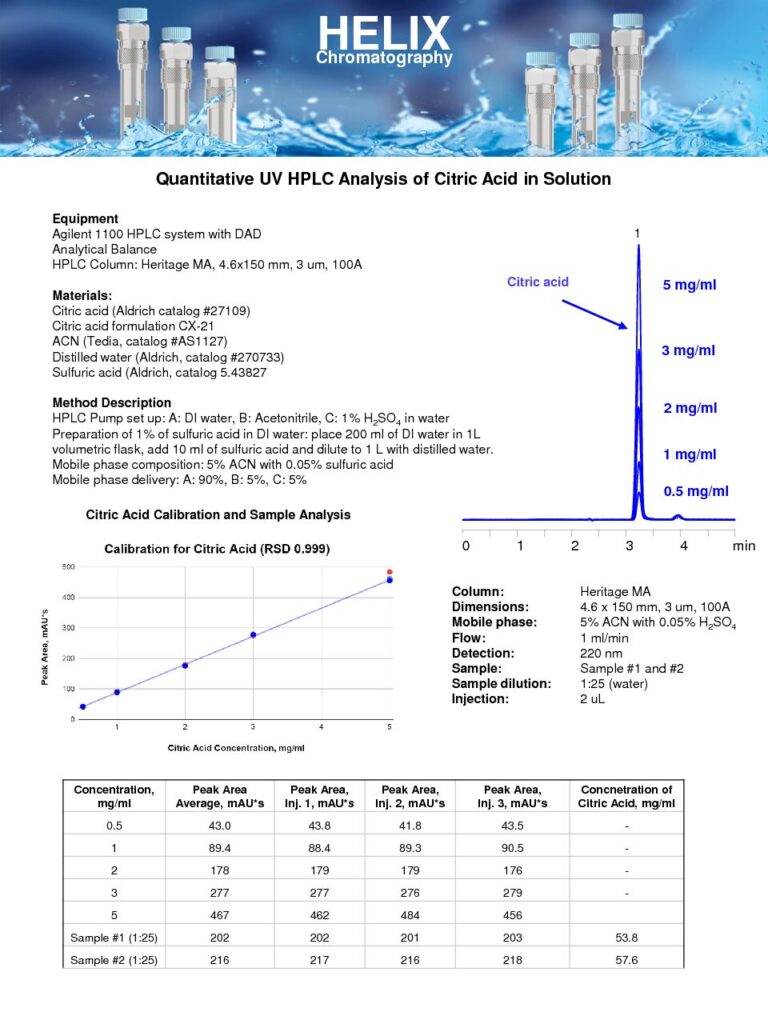 |
Quantitative UV HPLC Analysis of Citric Acid in Solution
The Quantitative UV HPLC Analysis of Citric Acid in Solution on Heritage MA mixed-mode column was developed as part of our HPLC Testing Services to determine the concentration of citric acid in a solution.Citric acid is a widely used compound with applications in various industries, including food and beverage, pharmaceuticals, and cosmetics. Its quantification in solution is crucial for quality control purposes, ensuring that the desired citric acid concentration is achieved and maintained.The mixed-mode approach allows for the efficient separation of citric acid from other components in the solution, enabling accurate measurement. The Heritage MA mixed-mode column enhances the retention and resolution of citric acid, ensuring reliable and reproducible results.
The quantitative analysis of citric acid in solution provides valuable information for product formulation, process optimization, and quality assurance. In the food and beverage industry, it helps ensure that the citric acid concentration in products such as juices, soft drinks, and sauces is within the desired range, contributing to the desired flavor profile and pH regulation. In pharmaceutical and cosmetic applications, it assists in maintaining the effectiveness and stability of formulations. |
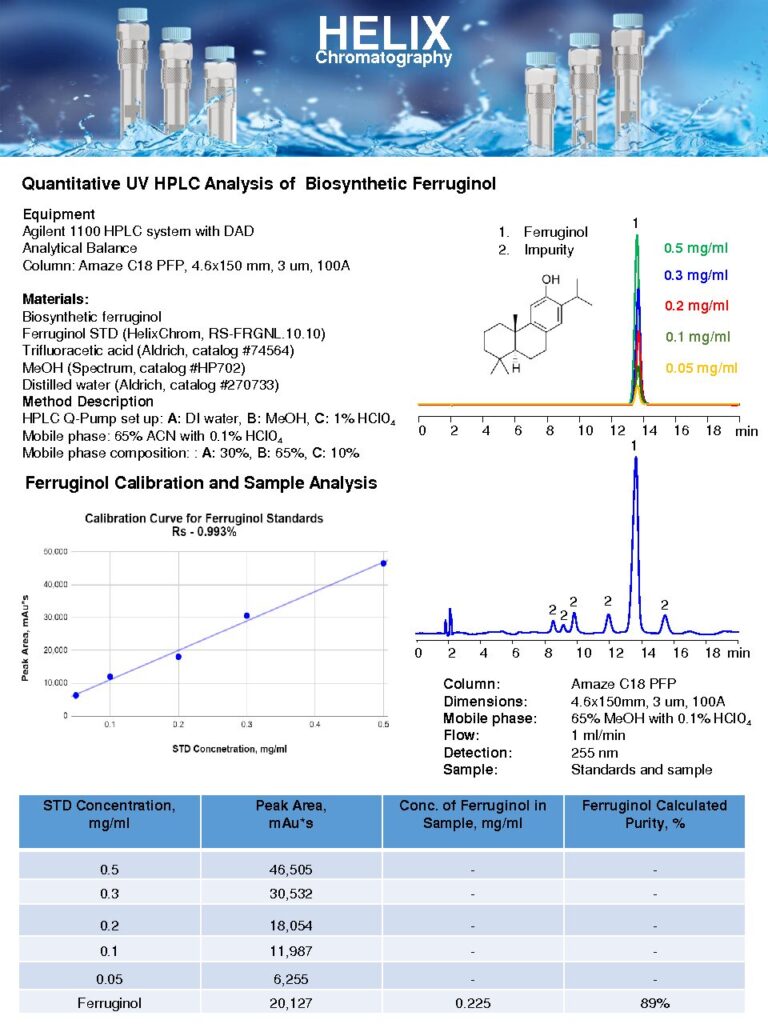 |
Quantitative UV HPLC Analysis of Biosynthetic Ferruginol
The Quantitative UV HPLC Analysis of Biosynthetic Ferruginol on Amaze C18 PFP column is a method used to measure the concentration of biosynthetic ferruginol in a sample.Biosynthetic ferruginol is a natural product derived from various plant sources and possesses significant biological activities. Its quantification is important for pharmaceutical research, drug development, and quality control purposes.The C18 PFP column offers both hydrophobic, polar interactions and pi-pi interactions allowing for enhanced retention and resolution of the target compound. The unique chemistry of this stationary phase provides a perfect shape for neutral basic and acidic analytes.
The quantitative analysis of biosynthetic ferruginol provides crucial information for assessing its purity, potency, and consistency. It aids in evaluating the efficiency of biosynthetic pathways, optimizing production processes, and ensuring the quality of the final product.
By employing UV HPLC analysis on the Amaze C18 PFP column, this method offers a sensitive and reliable approach for quantifying biosynthetic ferruginol. It contributes to the characterization, standardization, and quality control of this natural product, enabling researchers and manufacturers to explore its potential applications effectively. |