Mixed-mode chromatography is a separation technique that explores at least two interactions to retain and separate organic and inorganic compounds within one run. Depending on the design of the stationary phase there are different controllable mechanisms of interactions involved during the separation, including:
- Reversed-phase and cation-exchange (or anion-exclusion)
- Reversed-phase and anion-exchange (or cation-exclusion)
- Reversed-phase and pi-pi
- Reversed-phase, cation-exchange, and anion-exchange (cation-exclusion and anion-exclusion)
- Reversed-phase, pi-pi, and cation-exchange
- Reversed-phase, pi-pi, and anion-exchange
- HLIC and cation-exchange (or anion-exclusion)
- HILIC and anion-exchange (or cation-exclusion)
- HILIC cation- and anion-exchange
Benefits of Mixed-Mode Chromatography
Unique Properties:
- Alternative selectivity compared to RP, HILIC, normal phase and ion-exchange
- A significant increase of retention time for ionizable analyte
- Retention of polar and ionizable compounds without an ion-pairing reagent
- Analysis of organic and inorganic molecules in a single run
- Perfect peak shape for charged analytes due to ionizable groups on the surface
- Full compatibility with all detection techniques including mass spectrometry
- Five- to fifty-fold increase in loadability of ionic compounds in preparative chromatography
- Higher flexibility with a choice of diluent for analytes
- Ability to inject samples with high salt concentrations
Unprecedented Selectivity:
- Alternative selectivity compared to RP, HILIC, normal phase and ion-exchange
- Ability to separate isomers with high resolution and selectivity
- Ability to control retention time, resolution and separation by changing the amount of organic component, buffer nature, buffer concentration, and buffer pH
- Simultaneous analysis of drugs and corresponding counter-ions
- Ability to adjust retention time and order of elution for various compounds independently
- 2-D selectivity on one column
- The possibility of replacement of long gradient methods with a short isocratic run
Additional Benefits:
- Compatibility with 100% organic and 100% aqueous mobile phases
- Total resistance to stationary phase dewetting (phase collapse)
- More options for unique chemistry on the surface
- Robust stationary phases and methods
Mixed-Mode Stationary Phases Schematics and Usability
1. Reversed-Phase Bi-Modal Stationary Phases
n – carbon chain variations, m – multiple monomers, B – basic group in non-ionized state, BH+ – basic group in ionized state, AH – acidic group in non-ionized state, A – acidic group in ionized state, X – acidic or basic group, R – substitution in the aromatic ring
| Stationary Phase |
Type |
Applications Scope |
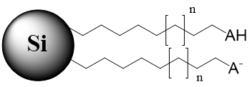 |
Bi-modal stationary phase with a hydrophobic chain and terminal ionizable acidic group (ionizable and non-ionizable states) |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic cations
- Basic and acidic isomers
|
 |
Bi-modal stationary phase with hydrophobic chain and the ionizable acidic group inside a hydrophobic chain (ionizable or non-ionizable state |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic cations
- Basic and acidic isomers
|
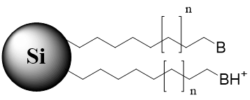 |
Bi-modal stationary phase with a hydrophobic chain and terminal ionizable basic group (ionizable or non-ionizable state) |
Retains and separates:
- Hydrophilic acidic compounds (organic and inorganic acids)
- Hydrophobic neutral compounds
- Hydrophobic basic compounds
- Inorganic anions
- Basic and acidic isomers
|
 |
Bi-modal stationary phase with a hydrophobic chain and ionizable basic group inside a hydrophobic chain (ionizable or non-ionizable state) |
Retains and separates:
- Hydrophilic acidic compounds (organic and inorganic acids)
- Hydrophobic neutral compounds
- Hydrophobic basic compounds
- Inorganic anions
- Basic and acidic isomers
|
 |
Bi-modal stationary phase with hydrophobic chain and an aromatic ring |
Retains and separates:
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Hydrophobic basic compounds
- Aromatic isomers
|
2. Reversed-Phase Tri-Modal and Quad-Modal Stationary Phases
| Stationary Phase |
Type |
Application Scope |
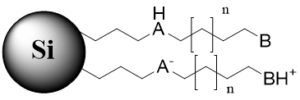 |
Tri-modal stationary phase with a hydrophobic chain and both basic and acidic groups (ionizable and non-ionizable states) |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophilic acidic compounds
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic cations and anions
- Basic and acidic isomers
|
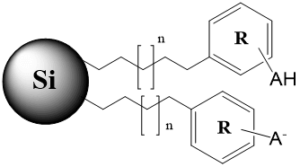 |
Tri-modal stationary phase with an alkyl chain, aromatic fragment and acidic group (ionizable and non-ionizable states) |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophilic acidic compounds
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic cations
- Basic and acidic isomers
|
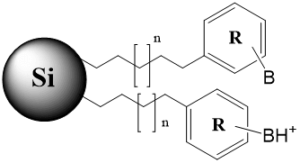 |
Tri-modal stationary phase with a hydrophobic chain, aromatic fragment and basic group (ionizable and non-ionizable states) |
Retains and separates:
- Hydrophilic acidic compounds
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic anions
- Basic and acidic isomers
- Aromatic neutral isomers
|
 |
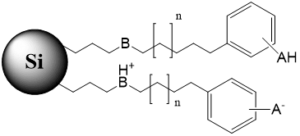
Tri-modal stationary phase with a hydrophobic chain, terminal ionizable group and polymeric bids of opposite charge |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophilic acidic compounds
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic cations and anions
- Basic and acidic isomers
|
 |
Quad-modal stationary phase with a hydrophobic chain, aromatic fragment, basic and acidic groups (ionizable and non-ionizable states) |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophilic acidic compounds
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic cations and anions
- Basic and acidic isomers
- Aromatic neutral isomers
|
3. HILIC Multi-Modal Stationary Phases
| Stationary Phase |
Type |
Application Scope |
 |
Bi-Modal stationary phase with a polar acidic group on the surface (ionizable and non-ionizable states) |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophilic acidic compounds
- Hydrophobic basic compounds
- Inorganic cations and anions
|
 |
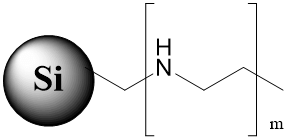
Bi-Modal stationary phase with a polar basic group on the surface (ionizable and non-ionizable states) |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophilic acidic compounds
- Hydrophobic acidic compounds
- Inorganic cations and anions
|
 |
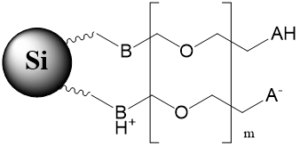
Bi-Modal stationary phase with polar basic polymer on the surface |
Retains and separates:
- Hydrophilic acidic compounds
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic anions
- Basic and acidic isomers
- Aromatic neutral isomers
|
 |
Tri-modal stationary phase with a hydrophilic chain, basic and acidic groups on the surface |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophilic acidic compounds
- Hydrophobic neutral compounds
- Hydrophobic acidic compounds
- Inorganic cations and anions
- Basic and acidic isomers
|
 |
Tri-modal stationary phase with polar basic and acidic groups |
Retains and separates:
- Hydrophilic basic compounds (amines, amino acids, amino sugars, pyridines, hydrophilic quaternary amines)
- Hydrophilic acidic compounds
- Inorganic cations and anions
|
Reversed-Phase and Cation-Exchange Columns from HELIX Chromatography
Reversed-phase cation-exchange mixed-mode columns usually carry hydrophobic alkyl chain as well as an acidic functional group. These columns are designed for separation of hydrophobic neutral, hydrophobic acidic, hydrophilic basic compounds, and acidic compounds. The retention time of compounds depends on the length of the carbon chain, the strength of the acid on the surface, mobile phase pH, amount of organic portion of the mobile phase, as well as the concentration of the buffer or acidic additives to the mobile phase. Stationary phase can carry either carboxylic acid or other acidic moieties. Such chemistry can be considered as attaching ion-pairing reagent to the surface of silica gel.
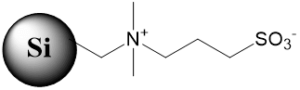
Coresep 100 Core-Shell Mixed-Mode Stationary Phase
Coresep 100 – is a reversed-phase cation-exchange column with core-shell particles. It has C12 carbon chain and carboxylic acid with pKa of 2. Acidic, basic, zwitterionic, and neutral compounds are separated based on RP/cation-exchange mechanisms.
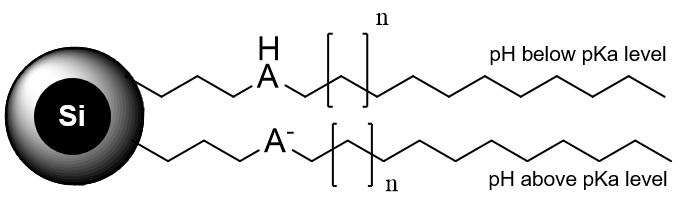 Fig. 1. Coresep 100 Core-Shell Mixed-Mode Stationary Phase Schematic
Fig. 1. Coresep 100 Core-Shell Mixed-Mode Stationary Phase Schematic
The retention time of basic and zwitterionic compounds can be effectively adjusted by changing the strength of the acid. The same concentration of organic or inorganic acid In the mobile phase can drastically increase the retention time of basic and zwitterionic analyte.
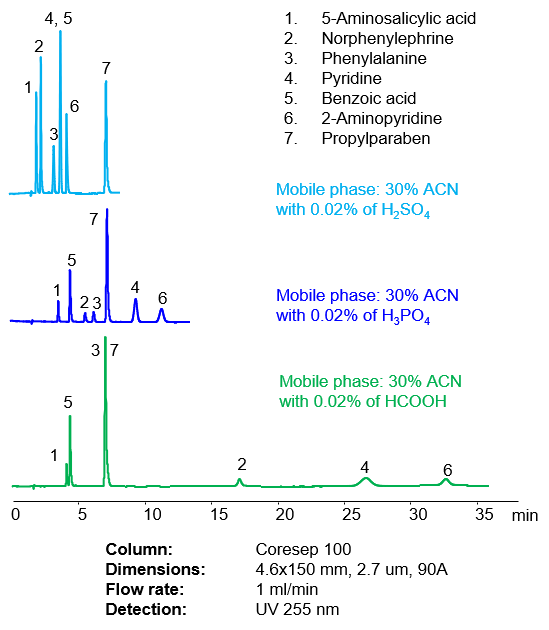 Fig. 2. Effect of various acids on retention of acidic, basic and neutral compounds in the mobile phase on Coresep 100 Core-Shell Mixed-Mode HPLC column
Fig. 2. Effect of various acids on retention of acidic, basic and neutral compounds in the mobile phase on Coresep 100 Core-Shell Mixed-Mode HPLC column
Replacing sulfuric acid with phosphoric acid increased retention time of basic and zwitterionic analytes by a factor of 1.5-3X. Further replacement of phosphoric acid with formic acid extends retention time another 2-4 fold. Each of the acids above creates a different pH and concentration of ions. Increase in pH of the mobile phase provides higher ionization of the stationary phase, thus supporting a stronger ion-exchange interaction that results in longer retention.
The presence of an acidic group on the surface allows to completely avoid the use of the ion-pairing reagent, making mixed-mode columns an ideal candidate for LC/MS methods.
Heritage MC Mixed-Mode Stationary Phase
Heritage MC is a reversed-phase cation-exchange column based on regular high purity silica gel. It has C18 carbon chain with weak acidic functionality (pH 3.8-4.0). Acidic, basic, zwitterionic, and neutral compounds are separated based on RP/cation-exchange mechanisms.
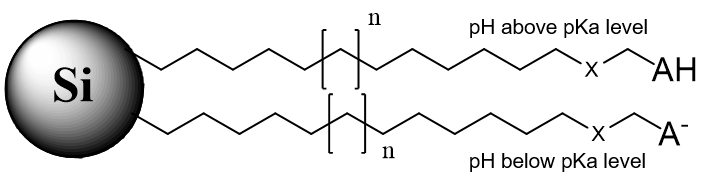 Fig. 3. Heritage MC Mixed-Mode Stationary Phase Schematic
Fig. 3. Heritage MC Mixed-Mode Stationary Phase Schematic
Presence of a weaker acidic moiety on the surface of silica gel can be used to shut down cation-exchange mechanism on the surface by using lower pH mobile phase. A lot of organic and inorganic acids can be used to create pH which is lower than pKa of the column. Such low pH (2-3), can suppress ionization of acidic fragment on the surface of silica gel and almost eliminates cation-exchange interaction. This approach can be helpful in prep isolation of the impurities.
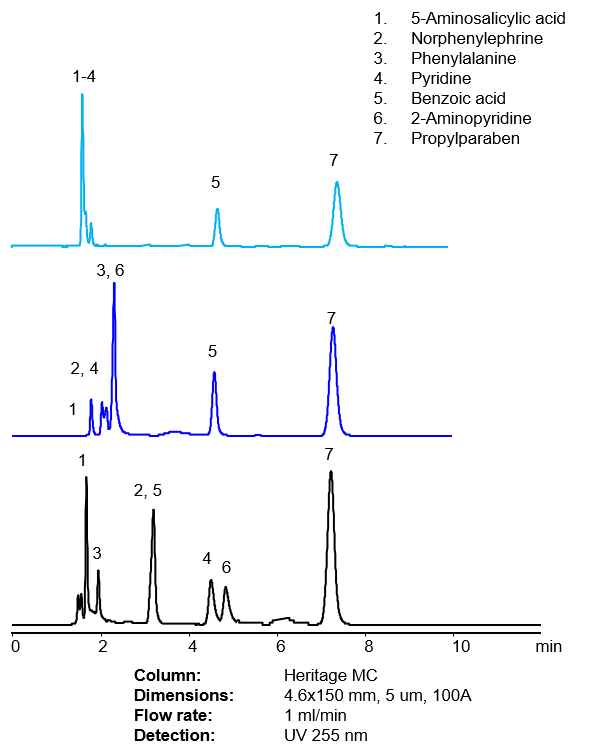 Fig. 4. Effect of various acids on retention of acidic, basic and neutral compounds in the mobile phase on Heritage MC Mixed-Mode HPLC column
Fig. 4. Effect of various acids on retention of acidic, basic and neutral compounds in the mobile phase on Heritage MC Mixed-Mode HPLC column
In order to retain basic compounds by cation-exchange mechanism, the analyte and stationary phase needs to be ionized. Cation-exchange on a Heritage MC column is more pronounced at pH above 4. At such pH, most of the basic compounds are ionized and will retain on the column based on reversed-phase and cation-exchange mechanisms. Increase in pH will improve cation-exchange mechanism.For zwitterionic analyte, an increase in pH will cause partial ionization of acidic fragment of the molecule, thus decreasing hydrophobicity and basicity of the molecule. The approach might be used to reduce retention time of amino acids with two or more basic groups.
Effective adjustment of pH can allow eluting compounds in bands based on the hydrophobic and ionic properties of the analytes.
Further increase in pH might suppress ionization of basic analytes, like pyridine, making them less basic but more hydrophobic.
Conclusion
Mixed-mode chromatography is an excellent choice for your method development and your everyday separation tasks. Unique selectivity combined with robust methods and columns will help you develop a reliable method. Never have co-elution or see peaks in the void unless you want it. Use mixed-mode columns when unique selectivity combined with mass spectrometry is required. The synergy of multiple interactions allows separating compounds which are very similar in nature. Multiple mechanisms can be applied to specific compounds independently letting you in one run to separate compounds with a drastic difference in properties.