Ion-Pairing Chromatography (IPC)
Ion-pairing chromatography was developed in 1973 to improve retention of polar ionizable analytes in reversed-phase chromatography. The cornerstone of the approach is the addition of surfactant type of material which carries the opposite to the analyte charge. Only one type of ion-pairing reagent can be used to retain either basic or acidic hydrophilic molecules.
For retention of basic hydrophilic molecules like simple amines or amino acids hydrophobic sulphonate, phosphonate or carboxylate can be used.
For retention of acidic hydrophilic compounds, like inorganic or organic acids, hydrophobic quaternary compounds are used.
Advantages of Ion-Pairing Chromatography
- IPC is a method for improving retention time of polar and charged analytes
- Two additional variables to affect selectivity
- Reproducible results
- Great choice of various length IP reagent from several manufacturers
- Peak shape of charged analytes can be greatly improved with IPC
- Columns usually demonstrate high efficiency and selectivity
- The use of IPC allows separating charged and neutral molecules in one run
Disadvantages of Ion-Pairing Chromatography
- IP methods are not compatible with MS
- More complex than simple reversed-phase chromatography
- Prep separation/isolation is problematic for most of IP methods
- Need for dedication of the column for IP methods
- Not easy or possible to separate analytes with opposite charge in one run
- Need for screening of various IP reagent to find conditions for separation
Mixed-Mode Chromatography as a Valuable Replacement of Ion-pairing Chromatography
The knowledge of limitations and advantages of ion-pairing chromatography helped to design stationary phases in which ion-pairing reagent-like compound is attached ton the surface of the silica imitating saturation of hydrophobic stationary phase with the ionic ion-pairing reagent.
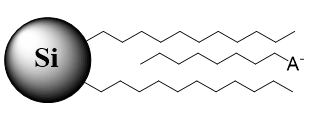 Fig. 1. Schematic of stationary phase with the anionic ion-pairing reagent used for retention of polar basic analytes
Fig. 1. Schematic of stationary phase with the anionic ion-pairing reagent used for retention of polar basic analytes
 Fig. 3. Schematic of stationary phase with the cationic ion-pairing reagent used for retention of polar acidic analytes
Fig. 3. Schematic of stationary phase with the cationic ion-pairing reagent used for retention of polar acidic analytes
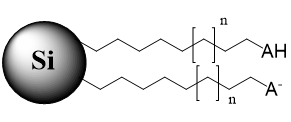 Fig. 2. Schematic of stationary phase with the immobilized anionic ion-pairing reagent used for retention of polar basic analytes
Fig. 2. Schematic of stationary phase with the immobilized anionic ion-pairing reagent used for retention of polar basic analytes
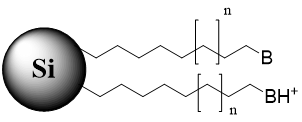 Fig. 4. Schematic of stationary phase with the immobilized cationic ion-pairing reagent used for retention of polar acidic analytes
Fig. 4. Schematic of stationary phase with the immobilized cationic ion-pairing reagent used for retention of polar acidic analytes